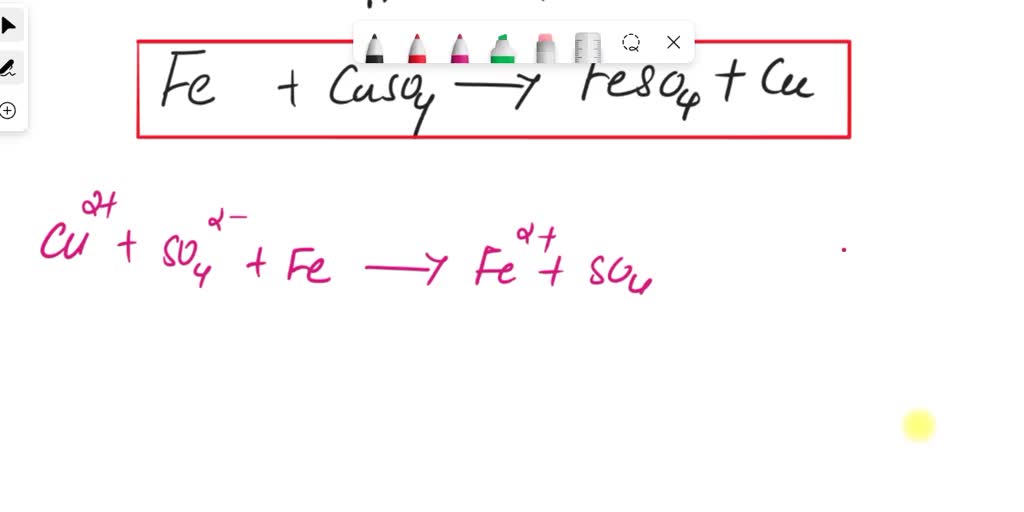Steel Wool And Hcl Reaction Equation . Web steel wool and vinegar reaction balanced equation. Be sure to include heat energy in the reaction. The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. When iron is exposed to oxygen, rust forms. Web write a balanced equation for the reaction of the steel wool with oxygen. Web answer reviewed 23 february 2023. Rusting (or oxidation) is a chemical. Allow about 5 minutes for the thermometer to record the temperature, then open the. Place the thermometer in the jar and close the lid. Web the balance sheet of the reaction between hydrochloric acid and iron is: Iron + hydrochloric acid → dihydrogen + iron ii. How are the products different from the reactants? What happened to the mass of the steel wool? Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust.
from www.numerade.com
Web steel wool and vinegar reaction balanced equation. Iron + hydrochloric acid → dihydrogen + iron ii. Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Web write a balanced equation for the reaction of the steel wool with oxygen. Rusting (or oxidation) is a chemical. What happened to the mass of the steel wool? Place the thermometer in the jar and close the lid. The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. Be sure to include heat energy in the reaction. When iron is exposed to oxygen, rust forms.
SOLVED reacting steel wool (iron) with copper (ii) sulfate what is
Steel Wool And Hcl Reaction Equation What happened to the mass of the steel wool? Web answer reviewed 23 february 2023. Iron + hydrochloric acid → dihydrogen + iron ii. Web steel wool and vinegar reaction balanced equation. Allow about 5 minutes for the thermometer to record the temperature, then open the. Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Web the balance sheet of the reaction between hydrochloric acid and iron is: Web write a balanced equation for the reaction of the steel wool with oxygen. Be sure to include heat energy in the reaction. The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. What happened to the mass of the steel wool? Rusting (or oxidation) is a chemical. When iron is exposed to oxygen, rust forms. How are the products different from the reactants? Place the thermometer in the jar and close the lid.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Hcl Reaction Equation Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Web steel wool and vinegar reaction balanced equation. When iron is exposed to oxygen, rust forms. Rusting (or oxidation) is a chemical. Allow about 5 minutes for the thermometer to record the temperature,. Steel Wool And Hcl Reaction Equation.
From www.pinterest.com
Steel Wool & Vinegar Chemical Reaction Science Experiments for Kids Steel Wool And Hcl Reaction Equation Web steel wool and vinegar reaction balanced equation. The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. Web the balance sheet of the reaction between hydrochloric acid and iron is: How are the products different from the reactants? Web when you soak the steel wool in vinegar it removes the protective coating of. Steel Wool And Hcl Reaction Equation.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Hcl Reaction Equation How are the products different from the reactants? Web write a balanced equation for the reaction of the steel wool with oxygen. Web answer reviewed 23 february 2023. Iron + hydrochloric acid → dihydrogen + iron ii. Rusting (or oxidation) is a chemical. What happened to the mass of the steel wool? Place the thermometer in the jar and close. Steel Wool And Hcl Reaction Equation.
From www.numerade.com
SOLVED reacting steel wool (iron) with copper (ii) sulfate what is Steel Wool And Hcl Reaction Equation Web answer reviewed 23 february 2023. Allow about 5 minutes for the thermometer to record the temperature, then open the. Web steel wool and vinegar reaction balanced equation. Rusting (or oxidation) is a chemical. Web write a balanced equation for the reaction of the steel wool with oxygen. Web when you soak the steel wool in vinegar it removes the. Steel Wool And Hcl Reaction Equation.
From www.transtutors.com
(Get Answer) II. Reaction of Iron (steel wool) with copper (II Steel Wool And Hcl Reaction Equation Web answer reviewed 23 february 2023. How are the products different from the reactants? The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. Web steel wool and vinegar reaction balanced equation. What happened to the mass of the steel wool? Rusting (or oxidation) is a chemical. Allow about 5 minutes for the thermometer. Steel Wool And Hcl Reaction Equation.
From studylib.net
Chemical Reaction Lab Steel Wool And Hcl Reaction Equation Allow about 5 minutes for the thermometer to record the temperature, then open the. Web answer reviewed 23 february 2023. How are the products different from the reactants? The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. Web when you soak the steel wool in vinegar it removes the protective coating of the. Steel Wool And Hcl Reaction Equation.
From www.chegg.com
Solved Write their balanced chemical equations in Steel Wool And Hcl Reaction Equation What happened to the mass of the steel wool? Web answer reviewed 23 february 2023. When iron is exposed to oxygen, rust forms. Web steel wool and vinegar reaction balanced equation. Place the thermometer in the jar and close the lid. The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. How are the. Steel Wool And Hcl Reaction Equation.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Hcl Reaction Equation Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Iron + hydrochloric acid → dihydrogen + iron ii. Place the thermometer in the jar and close the lid. When iron is exposed to oxygen, rust forms. What happened to the mass of. Steel Wool And Hcl Reaction Equation.
From dxoiwqfem.blob.core.windows.net
Vinegar And Steel Wool Chemical Equation at Deborah Hernandez blog Steel Wool And Hcl Reaction Equation Be sure to include heat energy in the reaction. Iron + hydrochloric acid → dihydrogen + iron ii. Web the balance sheet of the reaction between hydrochloric acid and iron is: Place the thermometer in the jar and close the lid. Web answer reviewed 23 february 2023. Allow about 5 minutes for the thermometer to record the temperature, then open. Steel Wool And Hcl Reaction Equation.
From ceubgelo.blob.core.windows.net
Steel Wool And Vinegar Reaction Balanced Equation at Dennis Turner blog Steel Wool And Hcl Reaction Equation The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. Web the balance sheet of the reaction between hydrochloric acid and iron is: Be sure to include heat energy in the reaction. Place the thermometer in the jar and close the lid. Iron + hydrochloric acid → dihydrogen + iron ii. Web steel wool. Steel Wool And Hcl Reaction Equation.
From upstartmag.squarespace.com
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Hcl Reaction Equation How are the products different from the reactants? Be sure to include heat energy in the reaction. Web answer reviewed 23 february 2023. Web the balance sheet of the reaction between hydrochloric acid and iron is: The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. Web steel wool and vinegar reaction balanced equation.. Steel Wool And Hcl Reaction Equation.
From makeupview.co
Chemical Makeup Of Steel Wool Makeupview.co Steel Wool And Hcl Reaction Equation How are the products different from the reactants? Be sure to include heat energy in the reaction. Web steel wool and vinegar reaction balanced equation. Iron + hydrochloric acid → dihydrogen + iron ii. Allow about 5 minutes for the thermometer to record the temperature, then open the. Web write a balanced equation for the reaction of the steel wool. Steel Wool And Hcl Reaction Equation.
From dbdalrymplemicrurus.z21.web.core.windows.net
Steel Wool And Vinegar Reaction Steel Wool And Hcl Reaction Equation Rusting (or oxidation) is a chemical. Iron + hydrochloric acid → dihydrogen + iron ii. Be sure to include heat energy in the reaction. Allow about 5 minutes for the thermometer to record the temperature, then open the. Web steel wool and vinegar reaction balanced equation. The steel wool and vinegar rust experiment is a great example to illustrate the. Steel Wool And Hcl Reaction Equation.
From www.slideshare.net
Replacement reactions Steel Wool And Hcl Reaction Equation Web steel wool and vinegar reaction balanced equation. Allow about 5 minutes for the thermometer to record the temperature, then open the. Place the thermometer in the jar and close the lid. Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Iron. Steel Wool And Hcl Reaction Equation.
From www.upstartmag.co.nz
Steel Wool Chemical Reaction Experiment for Kids — Upstart Magazine Steel Wool And Hcl Reaction Equation Web write a balanced equation for the reaction of the steel wool with oxygen. The steel wool and vinegar rust experiment is a great example to illustrate the highly exothermic. How are the products different from the reactants? Place the thermometer in the jar and close the lid. Web steel wool and vinegar reaction balanced equation. Be sure to include. Steel Wool And Hcl Reaction Equation.
From www.youtube.com
Combustion of Charcoal & Steel Wool YouTube Steel Wool And Hcl Reaction Equation What happened to the mass of the steel wool? Web the balance sheet of the reaction between hydrochloric acid and iron is: Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. How are the products different from the reactants? Web answer reviewed. Steel Wool And Hcl Reaction Equation.
From ceubgelo.blob.core.windows.net
Steel Wool And Vinegar Reaction Balanced Equation at Dennis Turner blog Steel Wool And Hcl Reaction Equation Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Web steel wool and vinegar reaction balanced equation. When iron is exposed to oxygen, rust forms. Allow about 5 minutes for the thermometer to record the temperature, then open the. Web write a. Steel Wool And Hcl Reaction Equation.
From teachingscience.us
Combustion Reaction Burning Steel Wool Teaching Science with Lynda R Steel Wool And Hcl Reaction Equation When iron is exposed to oxygen, rust forms. Web when you soak the steel wool in vinegar it removes the protective coating of the steel wool and allows the iron in the steel to rust. Web steel wool and vinegar reaction balanced equation. Iron + hydrochloric acid → dihydrogen + iron ii. Web the balance sheet of the reaction between. Steel Wool And Hcl Reaction Equation.